Engineering and physical science
Non-toxic Electrolytes for Li-ion Batteries
Li-ion batteries provide a portable light-weight means to power commonly used electronic devices with high energy density. Often, halogen-based electrolytes are used in Li-ion batteries in order to produce high power and energy densities. As a trade-off to these characteristics, many batteries have increased volatility and flammability issues that pose serious safety threats. Pairing different electrolytes with Li-ion cells can produce significant side effects ranging from safety, thermal stability and performance of the cell. To counteract these issues, additives are often used to reduce gas generation and mitigate flammability. Adversely, these additives pose a threat to cell performance; therefore, producing a need to replace current electrolytes in batteries with a new class of electrolytes that achieve efficient performance without the trade-off of safety.
The technology
Researchers at Virginia Commonwealth University have identified a new method of implementing halogen-free electrolytes in Li-ion batteries. The negative ion components of this new class of halogen-free electrolytes have stronger electron affinities than that of halogen atoms. Thus, batteries will become safer and environmentally friendly without disrupting the performance of the cell. Since the binding energy between Li cation and anion of the halogen-free electrolytes are small, Li-ions can easily transition from one electrode to the other, thereby improving performance. Additionally, since these electrolytes have low affinity to water, battery life is increased. Cell performance is also optimized without compromised adverse safety effects.
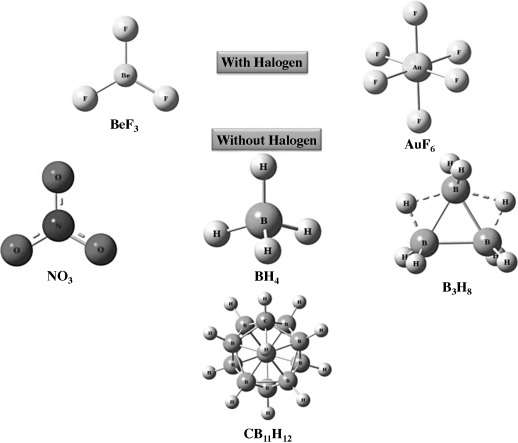
Figure 1. Optimized geometries of anions of four potential candidates for halogen‐free electrolytes. Also given for comparison are two other halogen‐containing anions that are not currently used in commercial applications.
