Medical Device
Stent Wash
Streamlining mechanical thrombectomy to save time and money
Mechanical thrombectomy treats large vessel occlusion (LVO), a cause of a cerebral infarct. In 2015, five randomized controlled trials were published in the New England Journal of Medicine showing significant benefit from the procedure, both establishing it as the standard of care and increasing the volume of procedures. One of the primary techniques to perform the procedure uses a stent retriever to capture and extract a thrombus from cerebral vessels. Often this technique requires multiple attempts, or “passes”, to recanalize the vessel. Reuse requires the device be removed with extracted thrombus portions entrapped on its stent tines. The retriever is then cleaned of thrombus debris prior to being used in additional passes. Stent retrievers are costly to purchase, and surgical use of multiple retrievers drastically increases medical costs. Therefore, a fast and repeatable method to mechanically clean these devices is expected to reduce procedure duration and overall cost.
The technology
VCU researchers have invented a device to rapidly and efficiently remove thrombus and blood products from stent retrievers or stents used during a mechanical thrombectomy or similar procedure. The device is handheld, containing a internal small chamber in which a stent retriever may be placed for cleaning. The device uses sterile syringes(s) to force sterile saline into the chamber. The design of the inflow and interior portion of the chamber maximize fluid agitation and propellant force for cleaning debris from the stent tines. The stent retriever is held within the chamber with the delivery wire extending out of the chamber on the surgical field. The device has a window that allows the operator to immediately visualize the status of the cleaning process.
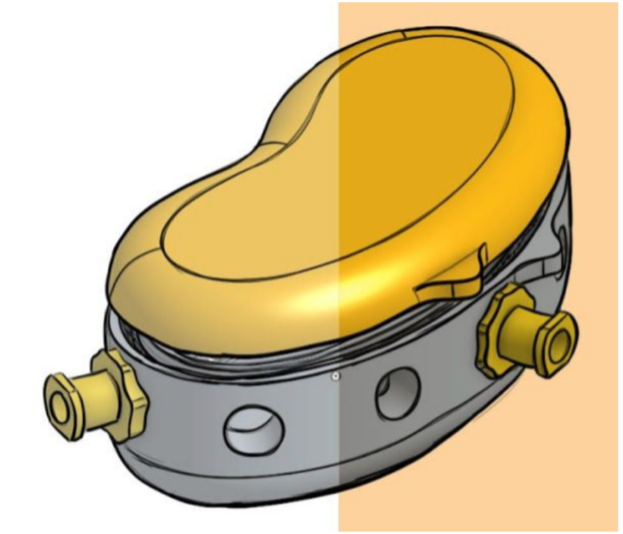
The device is made of a clear plastic to allow for visual assessment of the degree of cleaning accomplished. The design of the device allows for operation with one or two syringes, operated by hand, to provide the flushing flow of the cleaning solution. The device is amenable for sterile, single-use packaging as a standalone product or in a kit supplied with a stent retriever.
