Biomedical
Treatment for Enterovirus D68 Infection
The technology
Technology summary
Enterovirus D68 (EV-D68) is an acute respiratory illness contracted mainly by children that has a clinical manifestation that ranges from a benign upper respiratory infection to acute flaccid paralysis depending on the virus’s clade. Since 2014, there has been an upsurge of cases in North America, Europe, and Asia of EV-D68. Unfortunately, at the moment we have no antiviral treatment for this disease, only symptom based measures.
This treatment is a set of two double stranded RNA sequences, which targets all clades of EV-D68 and is non-toxic to host cells. It can be administered intra-nasally or through a nebulizer, which are proven effective, non-invasive routes of administration for children. It also has a low concentration in which it displays maximal inhibitory action.
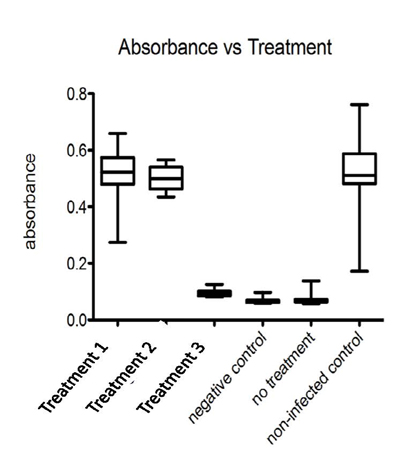
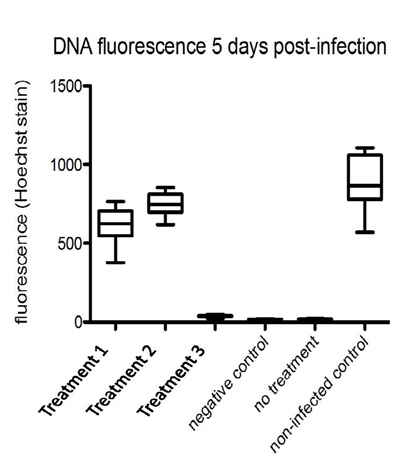
In the figure above, there are 3 treatments tested and 3 controls. In the infected without treatment cells, there is a large amount of cell death. Our treatment 1 & 2 recovers the cell viability to a level comparable to uninfected cells.
Technology status
In vitro data available
