Research tools
Human Imatinib Mesylate-Resistant Leukemia Cell Lines
Imatinib mesylate (Gleevec®) is currently the first line therapy for newly diagnosed Chronic Myelogenous Leukemia (CML) and Acute Lymphoblastic Leukemia (Ph+ ALL). However acquired resistance of cells to imatinib mesylate represents a significant clinical problem, in particular, mutations in the kinase domain of Bcr/Abl, which prevent drug binding and inhibitory activity, have been identified as the leading cause of resistance. This mutation has increasingly become the focus of research on CML and ALL, but currently there are no immortalized human leukemia (or other) cell line expressing the Bcr/Abl E255K mutation. All available cells of murine origin do not precisely recapitulate either the genetics or biology of human ALL or CML cells, and are therefore not ideal for such research.
The technology
By altering a commonly used in in vitro studies human ALL cell line, named BV173, researchers at Virginia Commonwealth University have generated an imatinib resistant cell line that contains the most frequently observed mutation (E255K) responsible for the drug resistance (Figure 1). This cell line is a unique resource in developing strategies capable of circumventing a clinically relevant and important form of the resistance to imatinib mesylate and potentially other tyrosine kinase (Bcr/Abl) inhibitors. In addition, it has been shown that this line is suitable for in vivo studies in immunocompromised mice, which adds to its value.
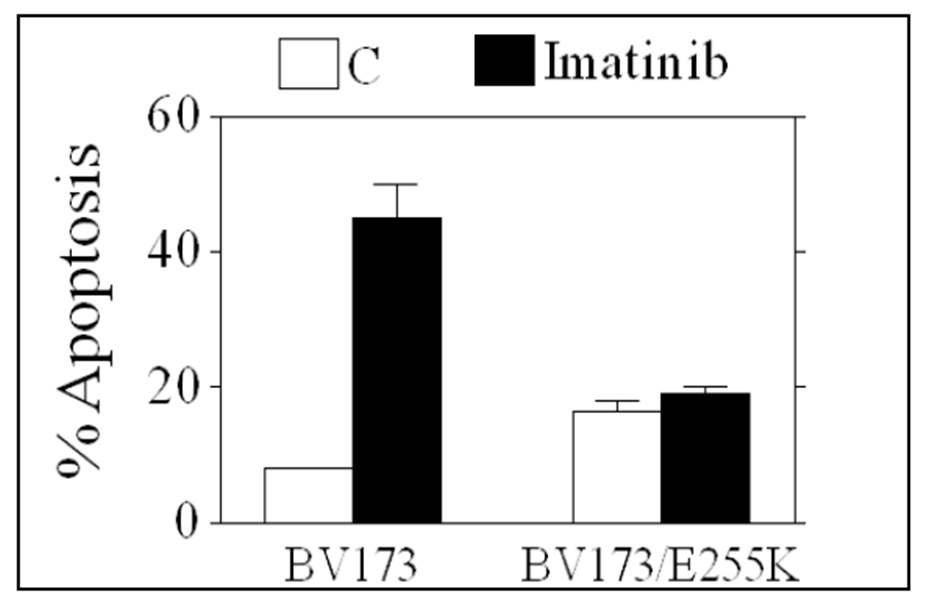
Figure 1. BV173: The bar labeled “C” has approximately 7% apoptosis, while the bar labeled “Imatinib” has approximately 45-50% apoptosis. BV173/E255K: “C” has approximately 15-20% apoptosis, while “Imatinib” has approximately 20% apoptosis.
